MRI/MRS as Non-Invasive Biomarker to Quantitatively Measure Disease Progression and Drug Efficacy
ImagingNMD is closely working with pharmaceutical companies to facilitate clinical trials of new drugs for various muscular dystrophies. We provide professional management of all MR related study activities and deliver precise MR outcome measurements. We are specialized in logistics of scan handling and tracking with extensive experience in skeletal and cardiac muscle imaging (MRI), spectroscopy (MRS), and the development of MRI/MRS biomarkers.
Our team are experts in acquisition and analysis of quantitative MRS and MRI outcomes, including muscle fat fraction (MRS and Dixon imaging), transverse relaxation time (T2), and cross-sectional area. Customized, advanced automatic analysis techniques are combined with expert supervision to maximize the precision of outcome measures and study power.
ImagingNMD’s Capabilities
- Communication with Sponsor and MR sites
- Project Management and oversight of MR sites
- Development of study specific MOPs and forms
- MR exam sequence development
- Training and support for MR study personnel
- Image quality control review
- MR technical support throughout study
- Data management and processing
- Data query processes
- Archival
Why Choose ImagingNMD UF as a Global Partner
We have an experienced team with a wealth of knowledge, experience with clinical trials, and collaboration with established MR sites. ImagingNMD is a world leader in developing and validating MR imaging biomarkers in Duchenne Muscular Dystrophy as well as Beckers and Limb Girdle Muscular Dystrophy.
Multiple MR experts with >80 years of combined experience
MR facilities chosen based on ability to successfully image children aged 4-18 years without sedation, following standardized procedures
Contracts with MR facilities strategically located in North America and Europe
Training and certification of MR sites and personnel to ensure optimal scanner performance and high-quality data collection
Provision of study materials including certified phantoms
Technical support to MR sites and advise on the choice of MRI/MRS metrics
Standardized SOPs and development of study specific MOPs and Forms
Centralized MR data reading, processing and management
MR site monitoring (remote and on-site) and MR data quality assurance
MRI
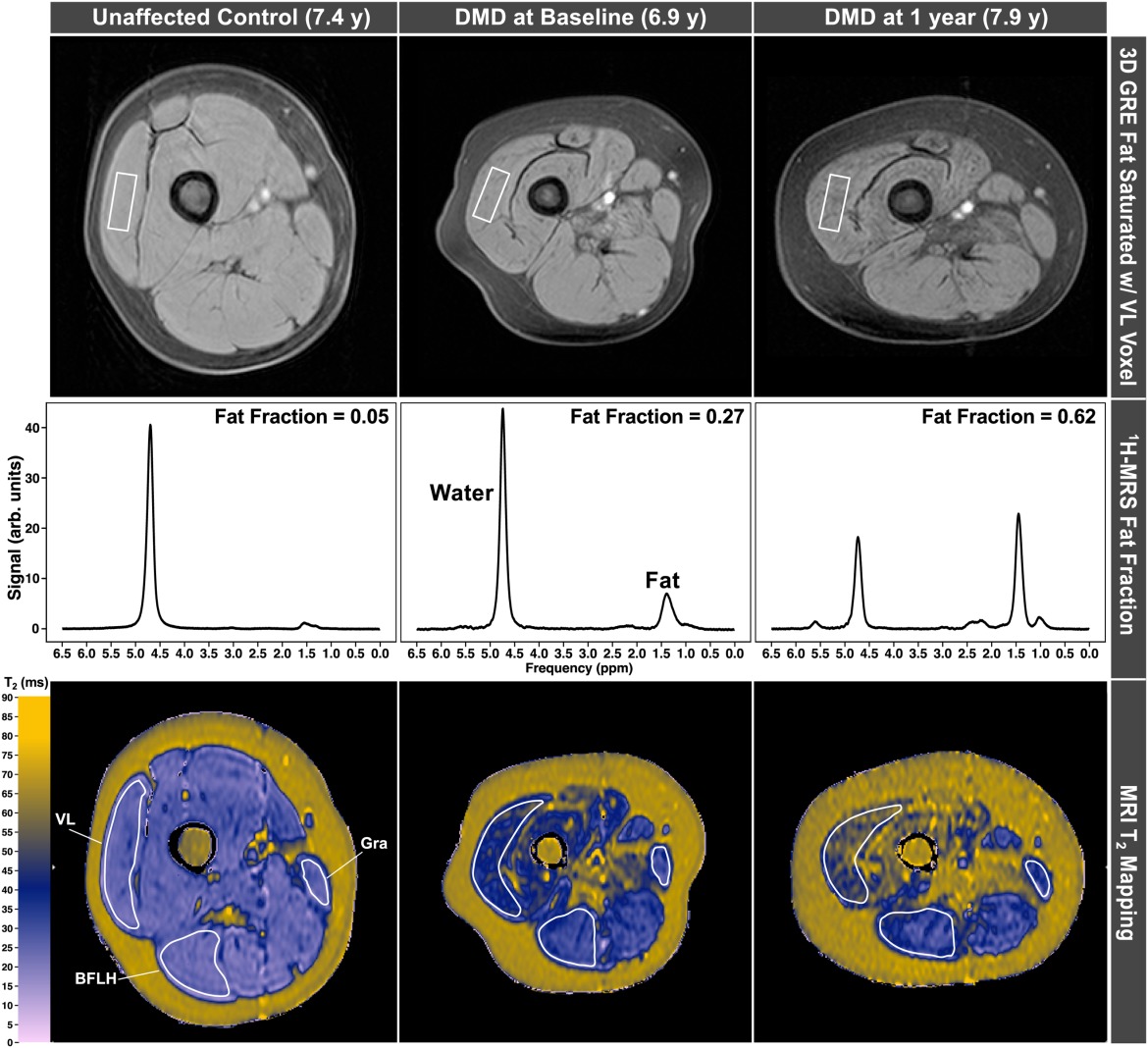
MRI is a non-invasive imaging method that provides detailed information about muscle structure and composition. MRI scanners generate images using magnetic fields and do not use ionizing radiation. Quantitative imaging biomarkers can accurately measure changes in muscle pathology.
MRS
MRS uses the same scanner and provides information about the chemical composition of tissues, including the fatty replacement of muscle, a valuable marker of disease progression in neuromuscular diseases.

Technical Expertise
Extensive Clinical Trial Experience with MRI/MRS Data as Endpoints
MRI/MRS biomarkers provide highly sensitive measures of muscle pathology and are valuable in the context of clinical trials.
As treatment options for neuromuscular diseases continue to expand, the need for imaging biomarkers in clinical trials is greater than ever. The ImagingNMD team at the University of Florida has the expertise and experience to meet all aspects of clinical trial needs for MR imaging. Our services range from expert consultation on protocol design, to implementation and management of data collection at specialized sites throughout the world, and refined quality control and analysis methods using custom proven algorithms for reliable and precise results.
- National and International Phase 1, 2 and 3 trials
- Excellent adherence to industry standards and local, national and international federal regulations
- Scan data quality control and rigorous training of MR imaging staff ensures optimal image quality and adherence to MR acquisition protocol
- Quantitative and qualitative real time image evaluation by MR experts.
- Highest standard of data analysis with blinded, automated analysis of MR data combined with expert supervision
- Secure, reliable data transfer from remote MR sites to the central ImagingNMD site at UF
- GCP, 21 CFR Part 11, and GDPR compliance with use of validated software tools and regular internal, sponsor and third party and QA audits
- ImagingNMD methodology has been published in high standard journals
Data Sharing Capabilities
Data Sharing
ImagingNMD has grown into one of the most comprehensive natural history studies in DMD and BMD and serves as a unique and valuable resource for muscular dystrophy communities. ImagingNMD shares MRI/MR derived data with accompanying demographic and functional information to facilitate the design of clinical trials. Raw data are collected from many different sources: Magnetic resonance imaging (MRI) and spectroscopy (MRS), genetic diagnoses, demographics, medical and medication history, strength and functional assessments, and biological samples. A summary of the available elements is provided in the Data Dictionary and a description can be found under the Cohort Demographics section.
Restricted Access
This data set will include de-identified DICOM image and spectroscopy files as well as tabular data files containing age, height, weight, functional assessment final scores, strength assessment scores, corticosteroid use status, ambulation status, and other information that can be included without compromising subject confidentiality. In accordance with our informed consent form, researchers will be required to apply for access to the data set by submitting the following information, which will be reviewed by an Executive Committee: Researcher name(s), institutional affiliation(s), and a brief proposal outlining how the data will be used. If shared data are used in subsequent publications the original funding source (AR056973) and the ImagingDMD network will need to be acknowledged and published methodology developed during the course of the study cited, as appropriate.